The number of protein structures being determined by cryo-electron microscopy is growing at an explosive rate.


A cryo-electron microscope at the Laboratory of Molecular Biology in Cambridge, UK.Credit: MRC Laboratory of Molecular Biology
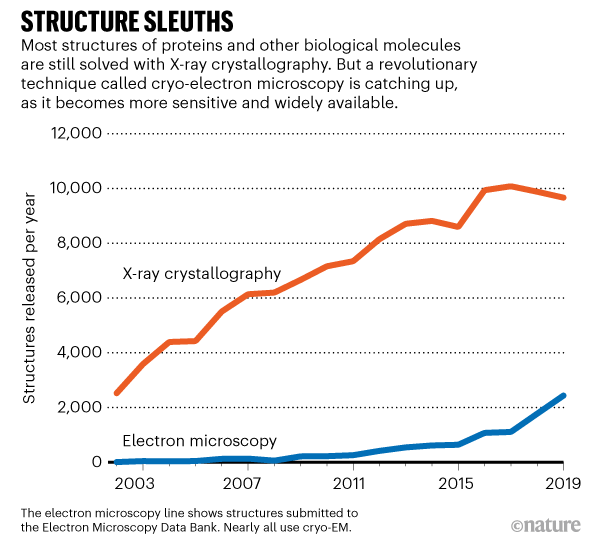
A revolutionary technique for determining the 3D shape of proteins is booming. Last week, a database that collects protein and other molecular structures determined by cryo-electron microscopy, or cryo-EM, acquired its 10,000th entry.
Submissions to the Electron Microscopy Data Bank (EMDB) — a popular repository for structures solved using electron microscopy — have increased exponentially in recent years, largely because of the explosive growth in the number of cryo-electron microscopes in labs worldwide (see ‘Structure sleuths’). The EMDB curates structures solved with other microscopy methods, but the vast majority use cryo-EM.

PDF version
MRC LMB Tritan Krios cryo-electron microscope.
A cryo-electron microscope at the Laboratory of Molecular Biology in Cambridge, UK.Credit: MRC Laboratory of Molecular Biology
A revolutionary technique for determining the 3D shape of proteins is booming. Last week, a database that collects protein and other molecular structures determined by cryo-electron microscopy, or cryo-EM, acquired its 10,000th entry.
Submissions to the Electron Microscopy Data Bank (EMDB) — a popular repository for structures solved using electron microscopy — have increased exponentially in recent years, largely because of the explosive growth in the number of cryo-electron microscopes in labs worldwide (see ‘Structure sleuths’). The EMDB curates structures solved with other microscopy methods, but the vast majority use cryo-EM.
The technique involves flash-freezing solutions of proteins or other biomolecules and then bombarding them with electrons to produce microscope images of individual molecules. These are used to reconstruct the 3D shape, or structure, of the molecule. Such structures are useful for uncovering how proteins work, how they malfunction in disease and how to target them with drugs.
For decades, structural biologists preferred to use X-ray crystallography, a technique that involves crystallizing proteins, pummelling them with X-rays and reconstructing their shape from the resulting tell-tale patterns of diffracted light. X-ray crystallography produces high-quality structures, but it’s not easy to use with all proteins — some can take months or years to crystallize, and others never crystallize at all. Cryo-EM doesn’t require protein crystals, but the technique languished because it tended to produce low-resolution structures — some scientists called it blobology.
Picture perfect
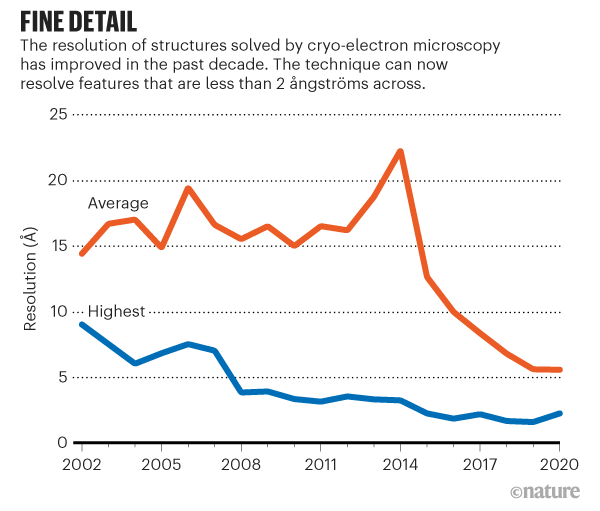
Breakthroughs in hardware and software in 2012–13 produced more sensitive electron microscopes and sophisticated software for transforming the images they captured into sharper molecular structures (see ‘Fine detail’). That paved the way for the current growth of cryo-EM, says Sjors Scheres, a structural biologist and specialist in the technique at the MRC Laboratory of Molecular Biology (LMB) in Cambridge, UK.
Richard Henderson, an LMB structural biologist who shared the 2017 Nobel Prize in Chemistry for his work developing the technique, says that even after these advances, growth was slow at first, because only a small number of labs had access to the equipment. But when they started using cryo-EM to produce detailed maps of molecules such as the ribosome — cells' protein-making machines — other scientists, as well as their institutions and funders, quickly took notice. “All the people who had invested in other things and made the wrong decisions, it took them a year to catch up,” says Henderson.

He estimates that by 2024, more protein structures will be determined by cryo-EM than by X-ray crystallography. Cryo-EM has already supplanted X-ray crystallography for one category of proteins that scientists are especially interested in — those embedded in cell membranes. Many such membrane-bound proteins are implicated in disease and serve as targets for drugs.
Advanced imaging
The structures of molecules determined by cryo-EM are also getting more detailed, thanks to continuing improvements in hardware and software, says Scheres.
Initially, the sharpest cryo-EM structures were of highly stable proteins that were used to test the limits of the technology. But Scheres has noticed that researchers are increasingly obtaining very high-resolution structures of medically important molecules, such as cell-membrane proteins, even though they tend to flop around.
“We’re now coming to the point where the easy samples have been done and people are looking at more complex problems,” says Ardan Patwardhan, a structural biologist at the European Molecular Biology Laboratory-European Bioinformatics Institute in Hinxton, UK, who leads the team that runs the EMDB.
Henderson expects the boom in cryo-EM structures to slow at some point. One factor that could sap growth, he says, is the high cost of the most powerful microscopes, which can exceed £5 million (US$7 million). They also cost thousands of pounds each day to run, and require specialized laboratories that minimize vibrations. Henderson is campaigning to convince firms to develop cheaper, but still useful, microscopes that could spread the technique even further. “At the moment, you cannot go wrong by putting more investment into cryo-EM,” he says.
Posted by Cláudio H. Dahne (Biological Sciences - UFC)
Comentários
Postar um comentário